Research Overview
We focuses on the vital field of reproductive and maternal-fetal health, recognizing the severe impact of infertility and gestation failure. Traditional basic research in these areas has been hindered by ethical and practical constraints. Our innovative approach uses human pluripotent stem cell (hPSC)-derived models to overcome these barriers, allowing us to explore the fundamental mechanisms inherent in the processes of fertilization, implantation, and early embryogenesis. Through targeted differentiation, organoid technologies, animal models, and cutting-edge genomic tools, we aim to unravel the pathology of these conditions, forging new therapeutic avenues, and working towards innovative treatments for infertility and gestation failure.
Our Projects
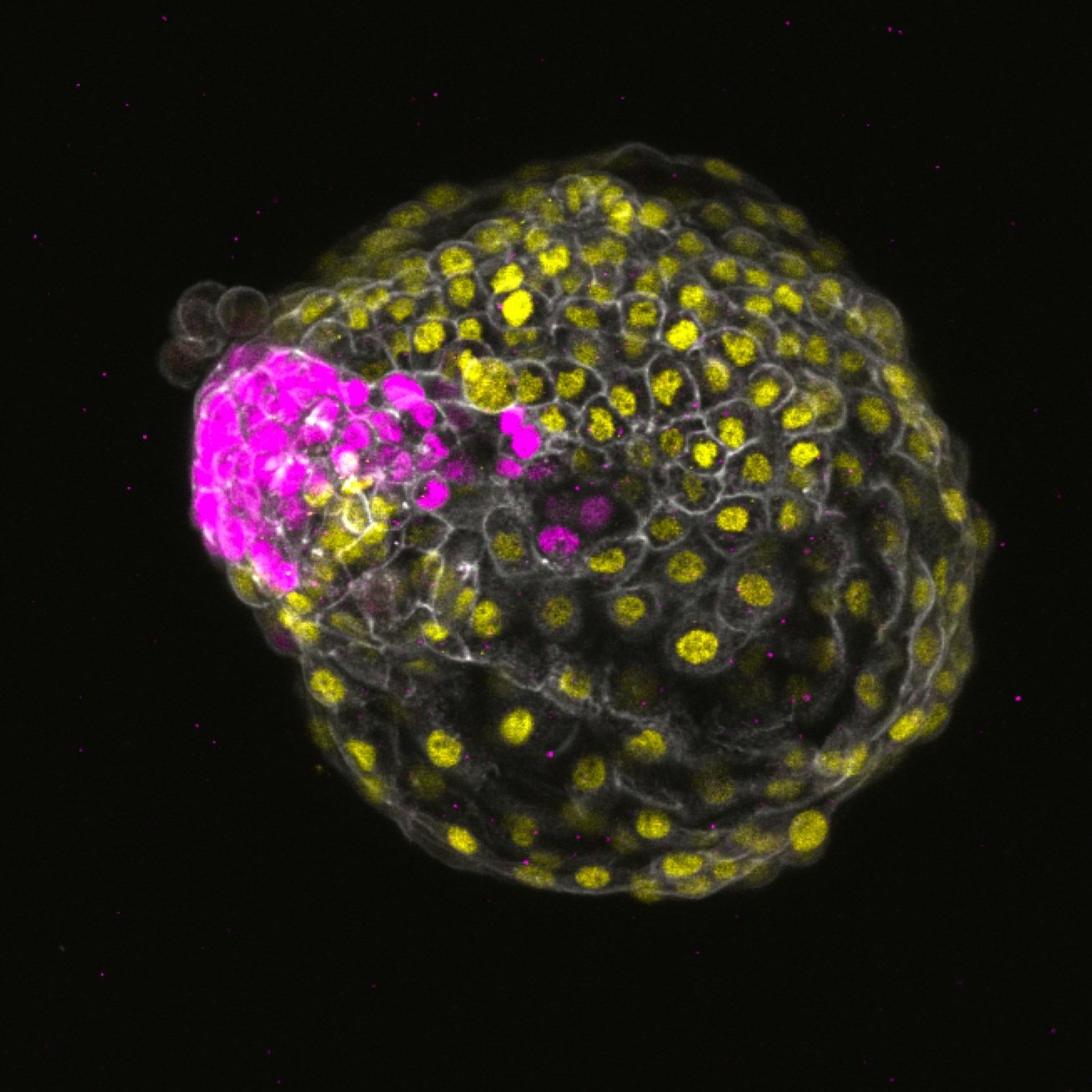
Characterization of aneuploidy, cell fate and mosaicism in early development
Chromosomal abnormalities in embryos is considered one of the major limitations to successful human reproduction and a significant cause of gestation failure. Our previous work demonstrated lineage-specific behaviors of aneuploidy (cells with chromosomal abnormalities) in early human development.
In this project, we will explore the following research questions:
What are the underlying mechanisms involved in cell fate determination and the elimination of aneuploidy?
Which signaling pathways are involved in the putative competition between normal and aneuploid cells within the context of mosaicism in early human development?
How do chromosomal abnormalities and instabilities manifest phenotypically in extraembryonic lineages?
To investigate these questions, we will utilize unique human pluripotent stem cell models, including micropatterned 2D gastruloids and blastoids. To ascertain in vivo relevance, we will also develop non-human primate models, specifically using marmosets. This will involve generating blastoids from naive-state marmoset embryonic stem cells.
This project is partially funded by the K99/R00 funding program from the National Institute of Child Health and Human Development (NICHD).
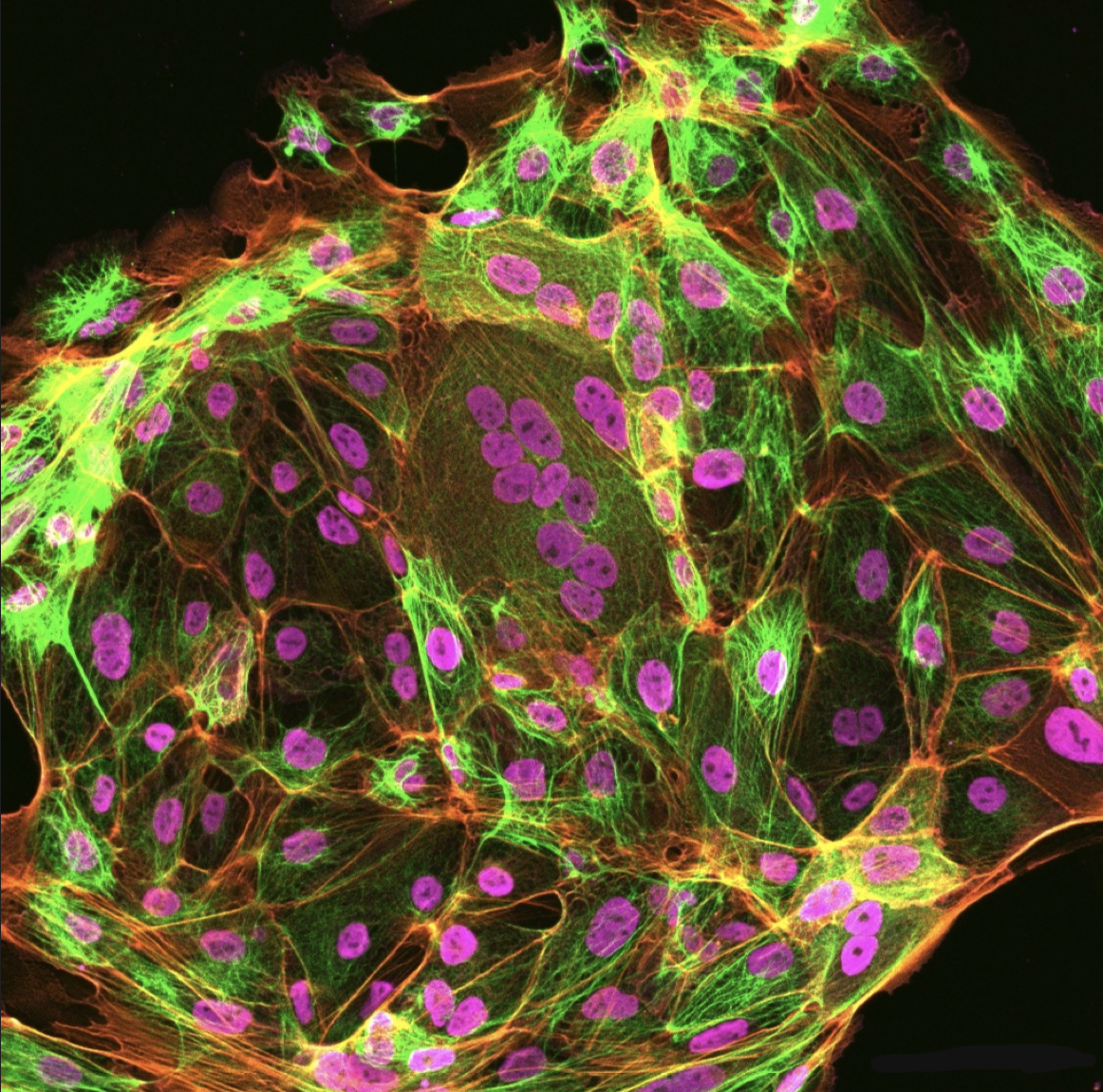
Modeling placentation anomalies using trophoblast stem cells
During the early stages of human pregnancy, placentation occurs, serving to anchor the developing embryo securely to the uterus. Improper placentation can lead to pregnancy loss or other serious complications such as preeclampsia, which can in turn result in neonatal illness or death. Recent advances have allowed for the derivation and cultivation of trophoblast stem cells (TSCs) from both placental issue and pluripotent stem cells. In this project, we aim to create in vitro models that recapitulate placentation process to investigate the cellular and molecular mechanisms involved in the development of placentation anomalies.
In this project, we will explore the following research questions:
What are the molecular cues that regulate the trophoblast differentiation and the development of placenta?
How does hormonal imbalances affect trophoblast differentiation process in early placentation?
What are the potential genetic factors that caused the abnormal placentation?
The goal of this project is to utilize in vitro models to investigate the molecular, hormonal, and genetic factors that influence trophoblast differentiation and contribute to abnormalities in early placental development, with the ultimate aim of translating these findings into improved diagnostic and therapeutic strategies for placental disorders.
Profiling the epigenetic regulation of cell fate transition in human embryos
Epigenetic regulations play a central role in governing the embryo development and somatic cell reprogramming. In this project, we will explore the role of epigenetic modifications in guiding the transformation of cells during early human embryonic development. By leveraging advanced genomics tools and cutting-edge embryonic extended culture technologies, we aim to map the epigenetic landscape of the early human differentiation. This will allow us to examine the dynamic changes in epigenetic marks during the critical stages of cell fate determination.
Our ultimate goal is to translate these findings into better diagnostic tools and more effective treatments for developmental diseases, and to provide critical insights into the process of human development.